The Complete Dummy's Genetics Guide for Idiots, Part Two
trickier concepts
This is the second article in a three-part series on genetics. In part one, we began with some basic concepts: looking at cells, the structure and replication of DNA, genes and what they do, and how cell division and genetic inheritance work. This article will delve a little deeper into some slightly more challenging concepts.
Let's go back to the genes. We have two copies of each gene, one from each parent. But what if these copies are different from one another? How does this change things? In fact, this is often the case. Most genes come in several different forms, called alleles. If genes were cars, then alleles could be Honda, Tesla or Lamborghini. Although affecting the same trait or traits, different alleles have differing DNA sequences, resulting in variations in the proteins they produce. Each of our 20,000 genes is located in a specific position (called a locus) on one of our chromosomes. All the alleles for a given gene can thus be found in the same position on the same chromosome as one another, but there can only be one of the possible alleles on any particular chromosome.
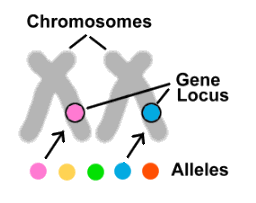
Now that we know what alleles are, let's look at the role they play in some common conditions.
Perhaps the simplest example in humans is albinism – a disorder resulting in a lack of melanin pigment in the skin, eyes and hair. One of the alleles for a gene that makes the protein required for melanin production codes for a defective version of the protein. Individuals who inherit this allele from both parents thus have no means of producing melanin and are albino. However, an individual with one defective allele and one good allele is still able to produce enough melanin to appear normal. So in this case, the good allele masks the defective allele, and geneticists say that the good allele is dominant, while the defective allele is recessive.1 Albinism is thus a recessive condition: it only shows up in people in whom both alleles are defective.
Remember that each parent gives one of their two alleles to each of their children, and that since this is determined at random, there's a ½ (50%) chance for a child to receive either allele (see figure, below). So, if two albinos have children, each child will also be albino (as the child will inevitably receive a defective allele from each parent). If an albino has a child with a non-albino, the albino parent will pass on one of their defective alleles to their child, but the non-albino will usually have two good alleles, and so the child will have one good allele and thus will be non-albino. However, note that in this case the child will carry one good allele and one defective allele, and so if the child subsequently had their own children and the other parent was albino, then each of these children would have a ½ chance of also being albino. And if two non-albino carriers (each having one good and one albino defective allele) had children, each child would have a ¼ chance of being albino (as shown in the figure below).
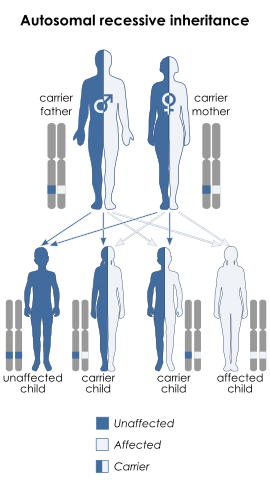 Autosomal recessive inheritance, for a trait such as albinism. In this case, both parents are carriers of the disease, so although they are themselves unaffected they can pass the trait on to their offspring. Note that if one parent was affected and one was a carrier, the affected parent would always pass on the disease allele, and half their children would be expected to be affected, based on which allele the carrier parent gave.
Autosomal recessive inheritance, for a trait such as albinism. In this case, both parents are carriers of the disease, so although they are themselves unaffected they can pass the trait on to their offspring. Note that if one parent was affected and one was a carrier, the affected parent would always pass on the disease allele, and half their children would be expected to be affected, based on which allele the carrier parent gave.
As well as recessive diseases (or non-disease traits), there are also diseases that are dominant. In these cases, the disease allele is dominant over the normal allele. This means that an individual will be affected by a disease even if they carry only one bad allele.
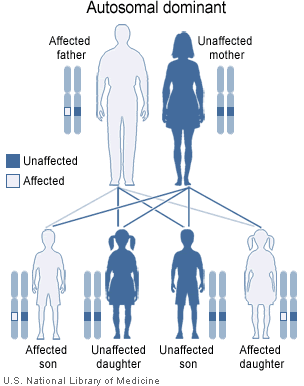 A generic example of autosomal dominance. Here, the father has a dominant disease allele and is affected. Each of his children has a 50% chance of receiving the disease allele and also being affected. Note that in this case the mother is free of disease alleles and so does not affect any of her children in relation to this particular disease (since she'll always pass on a healthy allele).
A generic example of autosomal dominance. Here, the father has a dominant disease allele and is affected. Each of his children has a 50% chance of receiving the disease allele and also being affected. Note that in this case the mother is free of disease alleles and so does not affect any of her children in relation to this particular disease (since she'll always pass on a healthy allele).
Dominant diseases tend to show up more frequently in a population than recessive diseases (because you only need one bad copy of the allele to be affected, rather than two, and so there are no unaffected carriers like there are with recessive diseases). This means that dominant diseases are generally either less severe than recessive diseases, or they show up later in life (otherwise these alleles would tend to eliminate themselves from a population). This is the case with Huntington disease, a lethal neurodegenerative disorder that is usually only clinically apparent at around age 40. By this age, many people will already have had children and may have passed on the Huntington allele to some or all of them. In this way, the Huntington allele does not eliminate itself from the population. Fortunately, as we'll consider in the final part of this series, there are now hundreds of diseases for which genetic screening is available, and individuals with a family history of such a disease can now be tested prior to having children.
What's actually going on at the molecular level that makes some traits dominant and some recessive? As we've learned, our genes produce proteins, and it turns out that a dominant allele is dominant because only one of the two alleles is required to produce sufficient protein. In the case of albinism, heterozygotes have a normal phenotype: although they have only a single functional allele, this is sufficient to produce enough protein to avoid albinism. We say that the normal allele is dominant over the albinism allele, because one normal allele produces enough protein to mask the effects of the albinism allele. On the other hand, in the case of Huntington's disease, it is the disease allele that is dominant over the normal allele. This is because two normal alleles are required to provide enough protein for normal function.
So far, we've considered only the effects of autosomal alleles. Things get more complicated when the gene is on the sex chromosomes. As we already learned, there are very few genes on the Y-chromosome, and those that are present are mostly involved in determining maleness. However, the X-chromosome does carry genes that code for many different traits. As with most genes, having a single correctly functioning gene on either of the two chromosomes is sufficient for normal function, so females carrying a disease allele will normally be unaffected. But remember, although females have two X-chromosomes, males have only one: if there is a mutation in an X-chromosome gene in a male, there is no 'backup' copy of the gene and so there will often be incorrect or non-existent functionality for the trait affected by that gene.
One familiar example of this is red/green colourblindness. The structures that allow us to perceive colours are the eye's retinal cones. Humans have three different types of cones, and the genes that control their development are on the X-chromosome. The different cone cells each contain a pigment sensitive to specific wavelengths of light, peaking in sensitivity for either red, green or blue light. Between them, the three types of cone cells cover the entire visible light spectrum, and can be used in combination, allowing us to distinguish millions of different colours. A mutation in one of the genes degrades or eliminates the ability of the person carrying the mutation to see the associated colour. In females, the effect of the mutation is negligible, since the second X-chromosome adds redundancy. However, in males the mutated gene is the only one of its kind, leading to the inability to see the colour in question. This is why colourblindness is much more common in males than in females.
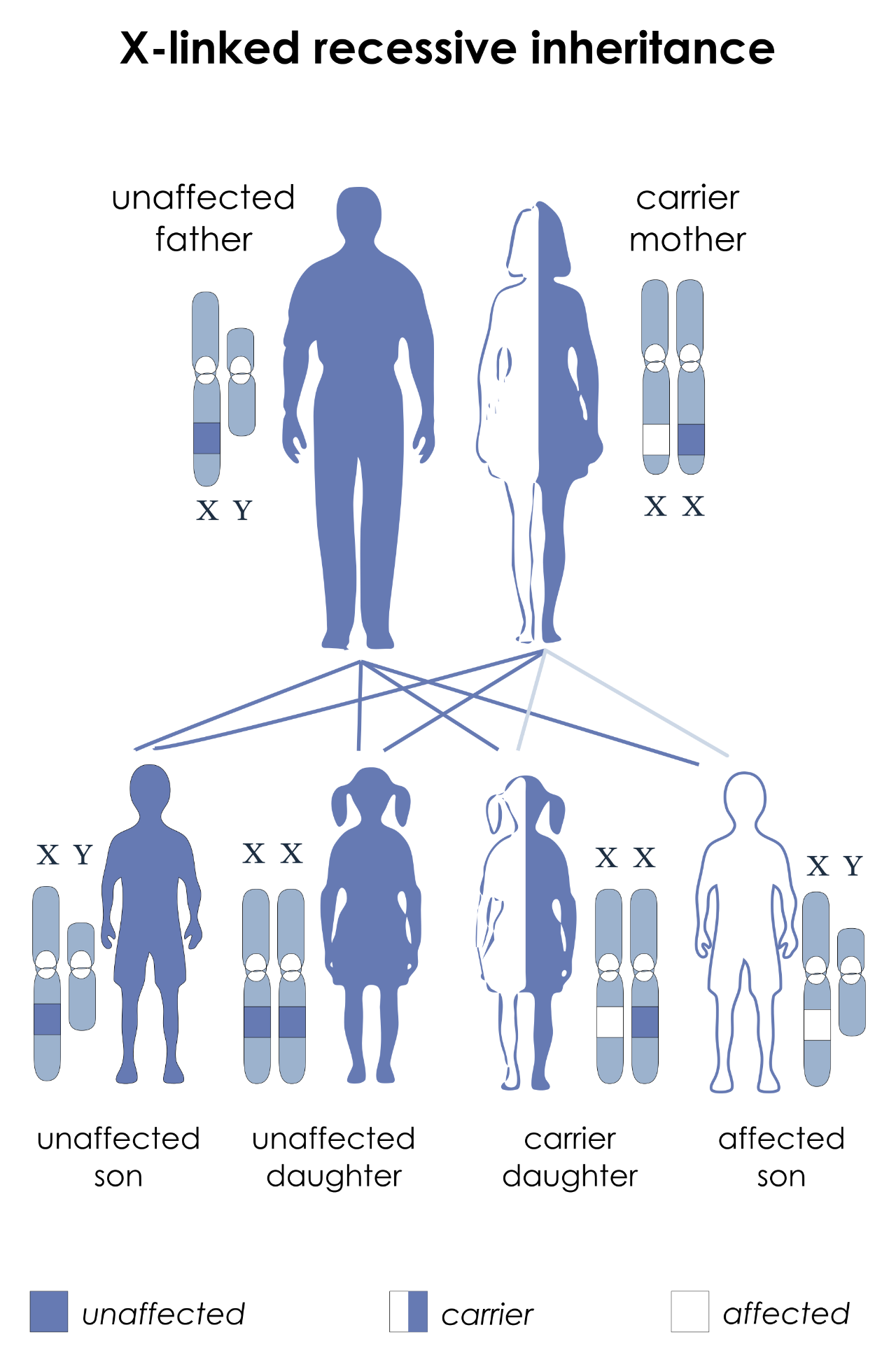 X-linked inheritance, for a trait such as red/green colourblindness. The gene in question is located on the X-chromosome. Here, the father has one normal gene on his X (so is not colourblind), while the mother has one normal copy and one mutated copy on her two X-chromosomes. The mother herself is unaffected (a carrier) because the disorder is recessive, but her children each have a 50% chance of inheriting the mutation. If this happens to any of her daughters, they will also become carriers, while any sons who inherit the chromosome with the mutation will be colourblind.
X-linked inheritance, for a trait such as red/green colourblindness. The gene in question is located on the X-chromosome. Here, the father has one normal gene on his X (so is not colourblind), while the mother has one normal copy and one mutated copy on her two X-chromosomes. The mother herself is unaffected (a carrier) because the disorder is recessive, but her children each have a 50% chance of inheriting the mutation. If this happens to any of her daughters, they will also become carriers, while any sons who inherit the chromosome with the mutation will be colourblind.
X-linked traits like colourblindness tend to skip generations, and with a little thought we can understand why. Suppose we have a normal-sighted female and a colourblind male, and this couple have children (see figure, below). The female parent has X-chromosomes with fully functional colour genes, so she won't contribute to colourblindness in any of her children. The male parent, however, being male has only one X-chromosome, and this chromosome has a non-functional colour-vision gene (which is why the male parent is colour-blind). The male children of this couple will receive their X-chromosome from their mother, and a Y-chromosome from their father, so their colour-vision will be normal. The female children will receive an X from both parents: a good X from their mother and a bad X from their father. Since one out of two good X-chromosomes is all that is required for normal colour vision, the female children (just like the male children) will all have normal colour vision. However, these female children will be carriers of colourblindness. Any children they subsequently have will have a 50% chance of receiving the X-chromosome carrying the colourblindness mutation, meaning that female children will also be carriers and male children will be colourblind. Hence, the colourblindness skipped from a male in the first generation to males in the third generation via one or more unaffected female carriers.
Read the previous paragraph through again if it wasn't quite clear.
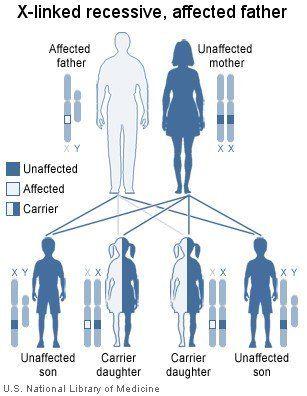 X-linked recessive disorders are passed down generations via female intermediaries. Here, we have an affected father who gives his X-chromosome containing the defective gene to all his daughters and none of his sons. His daughters, although themselves unaffected, can subsequently pass on the defective gene to their own children, and their male children who inherit the defective gene will be affected.
X-linked recessive disorders are passed down generations via female intermediaries. Here, we have an affected father who gives his X-chromosome containing the defective gene to all his daughters and none of his sons. His daughters, although themselves unaffected, can subsequently pass on the defective gene to their own children, and their male children who inherit the defective gene will be affected.
There are also X-linked dominant disorders. These are all rare and beyond the scope of this article.
As we know, genes operate by producing proteins, and the amount of protein produced depends on not only the specific alleles present, but also on the specific type of cell and which part of the body the cell is in. However, gene expression also varies over time: a developing human will have greatly differing gene expression as a foetus than it will as an infant, child or adult. Human development and gene expression are extremely large and complicated subjects, and we won't go into them in much detail here; let's just settle for a brief overview.
An example is illustrated by the enzyme lactase. Enzymes are proteins that catalyze (accelerate) reactions that occur in the body. Lactase is the enzyme that allows milk sugar (lactose) to be properly digested; without it, we develop the symptoms of lactose intolerance.
In nature, milk is only produced by nursing mothers (and milk production is one of the defining characteristics of mammals). Once an offspring is weaned, its production of lactase is greatly reduced, since there is no longer a need for it. However, with the advent of cattle domestication, some humans were able to produce non-human milk products as food on a regular basis and consume these throughout their lives. It turns out that individual humans have differing levels of lactase activity, and much of this difference can be explained by genetic variability: selective pressures in post-agricultural human populations led to the spread of a particular dominant mutation through those populations utilizing dairy production.2 This mutation causes lactase persistence – the ability to digest lactose beyond weaning due to high levels of lactase in the small intestine. Other populations, who didn't domesticate cattle, continue to down-regulate lactase after weaning, resulting in lactose intolerance. Prior to weaning, there is little difference in lactase expression between individuals, whether they have the mutation or not. However, in those not carrying a lactase-persistence mutation, regulatory proteins are produced following weaning that can bind to and interfere with the lactase gene, greatly reducing the gene's expression and thus leading to lactose intolerance.
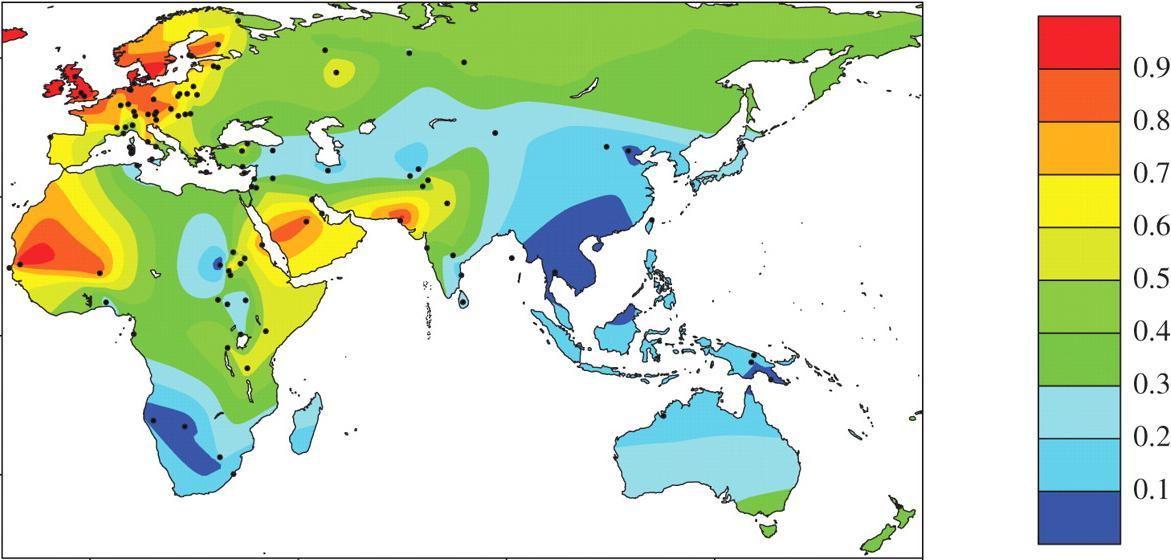 A map showing 'old world' lactase persistence frequencies. Lactase persistence mutations arose independently in Europe, sub-Saharan Africa and the Middle East, all of which regions developed dairy farming within the last few thousand years. As populations became more mobile and spread to various other parts of the world, they took the alleles with them.
A map showing 'old world' lactase persistence frequencies. Lactase persistence mutations arose independently in Europe, sub-Saharan Africa and the Middle East, all of which regions developed dairy farming within the last few thousand years. As populations became more mobile and spread to various other parts of the world, they took the alleles with them.
At the molecular level, genes are transcribed into mRNA via proteins that bind to a section of the DNA called the promoter. Other proteins (called polymerases) open up the DNA double helix and move along it, transcribing each letter of DNA into the corresponding letter of RNA. If a competing protein binds to the promoter before transcription can begin, this shuts down the expression of the gene. Thus, a cell can begin transcription by allowing an enhancer to bind to the DNA, or prevent transcription by allowing a repressor to bind. In those individuals exhibiting lactase persistence, the repressor protein for the lactase gene is not produced, which allows the continued production of lactase.
Other genes are regulated according to environmental conditions. For instance, if you ingest a poison, such as alcohol, your body responds by upregulating certain genes to metabolize the substance (in the case of alcohol, these include alcohol dehydrogenase, aldehyde dehydrogenase and fatty acid ethyl ester synthase, mainly in the liver and pancreas).
As well as affecting the amount of mRNA produced, there are many other forms of regulation: some involve modifying the way the mRNA is subsequently processed; some modify how much mRNA is allowed to exit the nucleus prior to translation into protein; others control the rate of translation into protein or the rate at which protein is degraded (see figure). The important point to bear in mind is that all these regulatory mechanisms serve to fine-tune the functioning of an organism based on a specific set of conditions.
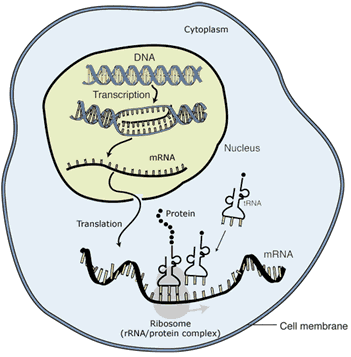 There are many stages at which protein production can be regulated. Starting inside the nucleus, cells can regulate whether, and how much, DNA is transcribed into mRNA; how mRNA is modified before leaving the nucleus; how much mRNA is allowed to leave the nucleus; whether and how many times a strand of mRNA is translated into protein; and how long a particular protein lasts until it is degraded.
There are many stages at which protein production can be regulated. Starting inside the nucleus, cells can regulate whether, and how much, DNA is transcribed into mRNA; how mRNA is modified before leaving the nucleus; how much mRNA is allowed to leave the nucleus; whether and how many times a strand of mRNA is translated into protein; and how long a particular protein lasts until it is degraded.
So far, we've thought of genes as acting independently of each other, but in fact this is rarely, if ever, the case.
We've just seen that the lactase gene is regulated by proteins that are produced by other genes, and which serve either to increase or decrease the gene's activity. But even this has greatly oversimplified what happens in the majority of cases. Take eye colour, which isn't simply determined by a single allele for each possible colour. Remember from an earlier example that pigmentation in eyes, hair and skin depends on a protein called melanin, which is produced by specialized body cells called melanocytes. Melanocytes are highly variable in the amount of melanin they can store, and this in turn leads to variability in the colour of eyes, hair and skin in individual humans. Eye colour is determined by the amount of melanin in the iris. The greater the amount of melanin, the more light is absorbed by the iris, and the darker the iris appears; blue eyes have low levels of pigment, whilst brown eyes have high levels.
Not only are there genes that affect the production of melanin, but also genes that affect its processing, storage and transport. In all, there are over one hundred genes that influence human pigmentation, at least eight of which are known to affect eye colour. Interactions between these genes all influence the amount of melanin that is taken up in the iris, and thus combine to determine eye colour. Furthermore, most of these genes will affect multiple other traits as well. This is generally the case; there are few genes that only affect a single trait. Rather, genes tend to work in overlapping networks, and their effects cascade down through genetic pathways and are in turn affected by other genes and feedback mechanisms.
The story gets yet more complicated when we consider the effects of the environment. The old argument of nature versus nurture to a large extent involves both sides arguing past one another. As we can probably appreciate, the genes that direct the building and functioning of our bodies are themselves affected by the outside world. It should be intuitive, for example, that the quality of nutrition that a child receives will affect the child's growth and development, both physical and intellectual. Diseases and infections also affect growth.3 There are many hundreds of genes that affect human growth, which not only interact with each other in large genetic networks, but are also affected by outside forces. Likewise, as we are all familiar with, melanin production is affected by light intensity. If you move to a high altitude, your body gradually increases the number of oxygen-carrying red blood cells, to counteract the lower amount of oxygen that is in the atmosphere. There are countless other examples we could enumerate.
So we have now moved far beyond our initial, overly-simplistic, view of genetics as one gene —> one trait. We know that most genes have many different versions, called alleles, and that some alleles are dominant over others. We also know that things work slightly differently when considering the sex chromosomes rather than autosomes, that gene regulation can give endless nuance with regard to the expression of genes and the proteins that are produced, that genes tend to have multiple effects, and that they interact with other genes and the environment.
In the final part of the series we're going to look at some topics in applied genetics, including chromosome variations, genetic mutation, cancer, selective breeding and the genetics of diseases.
Note that although we're looking at diseases here, the concepts of dominance and recessivity also apply to traits generally, some of which are benign variation rather than diseases.
Interestingly, the specific mutation varies by population. European, East African and North African populations each have a different lactase persistence allele.
Improvements to general levels of nutrition and health care over the last century or so in developed nations have led to dramatic increases in human growth, noticeable even from one generation to the next.